STUDYING PROCESSES OF ZrBe2 CORROSION IN HEAVY WATER VAPOR
https://doi.org/10.52676/1729-7885-2024-2-146-155
Abstract
This paper presents the results of an experiment to study the processes of ZrBe2 beryllyde corrosion when purging a sample with a stationary flow of Ar+D2O vapor-gas medium under thermal loads. ZrBe2 beryllide is considered as one of the promising materials for the use in various industries, science and technology, including thermonuclear energy. The interest in studying the processes of beryllides corrosion under conditions of purging with inert gases, with impurities of the water vapor of different isotopic compositions (H2O and D2O) is due to the need to understand the processes arising from such interaction.
The corrosion experiment with the ZrBe2 sample was carried out on a TGA/DSC 3+ synchronous thermogravimetric analysis and differential scanning calorimetry device manufactured by Mettler-Toledo (Switzerland), complete with a Pfeiffer ThermoStar quadrupole mass spectrometer, in the temperature range from 100 °C to 1200 °C. Crushed, industrially manufactured zirconium beryllide produced by Ulba Metallurgical Plant, JSC (Ust-Kamenogorsk, Kazakhstan) was chosen as the object of study.
As a result of the analysis of the experimental data, a mechanism for the interaction of the heavy water vapor with zirconium beryllide has been proposed and an equation has been obtained for determining the ZrBe2 corrosion rate constant during the interaction with heavy water vapor during purging with a vapor-gas mixture (Ar+D2O):
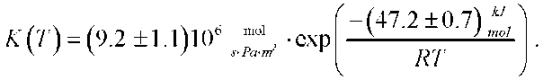
About the Authors
Yu. N. GordiyenkoКазахстан
Kurchatov
T. V. Kulsarov
Казахстан
Kurchatov
V. S. Bochkov
Казахстан
Kurchatov
Zh. A. Zaurbekova
Казахстан
Kurchatov
Yu. V. Ponkratov
Казахстан
Kurchatov
K. K. Samarkhanov
Казахстан
Kurchatov
S. V. Udartsev
Казахстан
Ust-Kamenogorsk
References
1. Nakamichi M., Yonehara K., Wakai D. Trial fabrication of beryllides as advanced neutron multiplier // Fusion Eng. Des. – Volume 86. – 2011. – P. 2262–2264. https://doi.org/10.1016/j.fusengdes.2011.03.009
2. Kim J.H., Nakamichi M. Characterization of modified Be13Zr beryllide pebbles as advanced neutron multipliers // Fusion Eng. Des. – Volume 146, Part B. – 2019. – P. 2608–2612. https://doi.org/10.1016/j.fusengdes.2019.04.054
3. Mukai K., Kasada R., Kim J.H., Nakamichi M. Electronic descriptors for vacancy formation and hydrogen solution in Be-rich intermetallics // Acta Mater. – Volume 241. – 2022. – Аrt 118428. https://doi.org/10.1016/j.actamat.2022.118428
4. Singh D.J., Gupta M. Anomalous structural behavior and electronic structure in ZrBe2Hx: Density functional calculations // Phys. Rev. B.– Volume 76. – 2007.– Art. 075120. https://doi.org/10.1103/PhysRevB.76.075120
5. Shein I.R., Medvedeva N.I., Ivanovskii. A.L. The band structures of superconducting MgB2 and the isostructural compounds CaGa2, AgB2, AuB2, ZrBe2, and HfBe2 // Phys. Solid State. – Volume 43. – 2001. – P. 2213-2218. https://doi.org/10.1134/1.1427944.
6. Goesten M.G. Be–Be π-bonding and predicted superconductivity in MBe2 (M= Zr, Hf) // Angew Chem Int Ed Engl. – 61(43). – 2022. –P. e202114303. https://doi.org/10.1002/ange.202114303.
7. Paine R.M.,.Stonehouse A.J, Beaver W.W. High temperature oxidation resistance of the beryllides // Corrosion. – Volume 20 (10). – 1964. – P. 307t-t313. https://doi.org/10.5006/0010-9312-20.10.307t.
8. Fleischer R.L., Zabala R.J. Mechanical properties of high-temperature beryllium intermetallic compounds // Metall. Trans. A. – Volume 20. – 1989. – P. 1279–1282. https://doi.org/10.1007/BF02647411
9. Miyamoto M., Sugimoto Y., Nishijima D., Baldwin M.J., Doerner R.P., Zaloznik A., Kim J.H., Nakamichi M. Comparative study of surface modification and D retention between beryllium and beryllides under high flux plasma exposure //Nuclear Materials and Energy. – Volume 27. – June 2021. – 101014. https://doi.org/10.1016/j.nme.2021.101014.
10. Mishima Yoshinao, Yoshida Naoaki, Takahash Heishichiro, Ishida Kiyohito, Kawamura Hiroshi, Iwadachi Takaharu, Shibayama Tamaki, Ohnuma Ikuo, Sato Yoshiyuki, Munakata Kenzo, Iwakiri Hirotomo, Uchida Munenori Present status of beryllides for fusion and industrial applications in Japan // Fusion Engineering and Design. – Volume 82, Issue 1. – 2007. – P. 91–97. https://doi.org/10.1016/j.fusengdes.2006.07.091.
11. Kawamura H, Takahashi H, Yoshida N, Mishima Y, Ishida K, Iwadachi T, Cardella A., van der Laan J.G, Uchida M., Munakata K., Sato Y., Shestakov V., Tanaka S. Resent status of beryllide R&D as neutron multiplier // Journal of Nuclear Materials. – Volumes 329–333, Part A. – 2004. – P. 112–118. https://doi.org/10.1016/j.jnucmat.2004.04.297.
12. Kinga D.J.M., Knowles A.J., Bowden D., Wenman M.R., Capp S., Gorley M., Shimwell J., Packer L., Gilbert M.R., Harte A. Review High temperature zirconium alloys for fusion energy // Journal of Nuclear Materials. – Volumes 559. – 2022. – 153431. https://doi.org/10.1016/j.jnucmat.2021.153431.
13. Forty B.A., Karditsas P.J. Uses of zirconium alloys in fusion applications // Journal of Nuclear Materials Volumes 283–287, Part 1. – December 2000. – P. 607-610. https://doi.org/10.1016/S0022-3115(00)00146-X
14. Nakamichi, M., Kim J. H., & Ochiai K. Beryllide pebble fabrication of Be–Zr compositions as advanced neutron multipliers // Fusion Engineering and Design. Volumes 109-111. – 2016. – P. 1719–1723. https://doi.org/10.1016/j.fusengdes.2015.10.018
15. Kim J.-H., & Nakamichi M. Anomalous oxidation behavior in a zirconium beryllium intermetallic compound // Journal of Nuclear Materials. – Volume 519. – 2019. – P. 182–187. https://doi.org/10.1016/j.jnucmat.2019.03.042
16. Ervin G., Nakata M. M. J. \ Electrochem. Soc. – Volume 110. – 1963. –P. 1103–1110
17. Yergazy Kenzhin, Inesh Kenzhina, , Timur Kulsartov, Zhanna Zaurbekova, Saulet Askerbekov, , Yuriy Ponkratov, Yuriy Gordienko, Alexandr Yelishenkov, Sergey Udartsev Study of hydrogen sorption and desorption processes of zirconium beryllide ZrBe2. // J. Nuclear Materials and Energy. – Volume 39. – 2024. – 101634 https://doi.org/10.1016/j.nme.2024.101634 101634
18. Okamoto H., Tanner L.E, and Abriata J.P. “The Be-Zr (Beryllium-Zirconium) System,” Phase Diagrams of Binary Beryllium Alloys // ASM International, Metals Park, OH. – 1987. – P. 223–229. https://doi.org/10.1007/s11669-007-9179-6
19. Ponkratov Yu.V., Bochkov V. S., Samarkhanov K.K., Karambayeve I.S. Methodology of Corrosion Testing of Nuclear and Fusion Reactors Materials Using TGA/DSC and MS Complex Techniques. // Eurasian Chemico-Technological Journal. – Volume 21 (1). – 2019. – P. 35–40. https://doi.org/10.18321/ectj787
20. Davydov D.A, Kholopova O.V. Formation and degradation of beryllium oxide films // Questions of atomic science and technology. Series Thermonuclear Fusion. – issue 2. – 2010. – P.39–49.
21. Arthur T. Motta,1 Adrien Couet,1 and Robert J. Comstock Corrosion of Zirconium Alloys Used for Nuclear Fuel Cladding // Annu. Rev. Mater. Res. – Volume 45. – 2015. – P. 311–43. https://doi.org/10.1146/annurev-matsci-070214-020951
Review
For citations:
Gordiyenko Yu.N., Kulsarov T.V., Bochkov V.S., Zaurbekova Zh.A., Ponkratov Yu.V., Samarkhanov K.K., Udartsev S.V. STUDYING PROCESSES OF ZrBe2 CORROSION IN HEAVY WATER VAPOR. NNC RK Bulletin. 2024;(2):146-155. (In Russ.) https://doi.org/10.52676/1729-7885-2024-2-146-155
JATS XML

















